One of the most challenging questions to ask right now is, “Are you vaccinated?” Public Trust in Government has been in decline for decades and “vaccine passports” to demonstrate proof of COVID-19 vaccination face vocal opposition from state legislatures, the ACLU, and the EFF. Businesses and healthcare providers alike are left wondering what tools are available to help them chart a return to normalcy.
 The good news is that for about half of people in the United States right now, the answer is to the vaccine question is “yes.” Post-jab, many received a paper card with handwritten information or hastily applied stickers containing a lot number and immunization date(s). For some settings, the honor system and cardstock might be enough, but not in every case; most don’t realize that there is something better.
The good news is that for about half of people in the United States right now, the answer is to the vaccine question is “yes.” Post-jab, many received a paper card with handwritten information or hastily applied stickers containing a lot number and immunization date(s). For some settings, the honor system and cardstock might be enough, but not in every case; most don’t realize that there is something better.
Let people print their own COVID-19 vaccination cards!
The ACLU agrees. From a healthcare equity standpoint, any solution must have a paper option. Not everyone carries a smartphone, and digital-only solutions will only exacerbate existing disparities.
To be successful, a strategy needs to bridge many divides. Blending the concepts of data privacy and ease of interoperability isn’t intuitive or easy, but it is possible. Helping analog reality stay connected to an increasingly digital world demands innovation. We can empower individuals to share proof of their COVID-19 vaccination—with whomever they choose—without creating a massive, centralized database.
What if we took the exact same information that’s shown on a vaccination card and made it machine-readable? QR Codes, like the ones you can scan for a restaurant’s online menu, or the one you show when checking in to your flight, are convenient and ubiquitous. They can be stored digitally on a phone or printed and carried in a wallet.
Since the introduction of Meaningful Use in 2009, healthcare organizations have been encouraged to report the vaccines that they administer to their state’s Immunization Information System. Louisiana and California were among the first states to use this reporting infrastructure to make printable barcodes, joining a growing number of public and private entities who are empowering people to share health-related information. This same infrastructure was used to record almost every dose of the coronavirus vaccines given in the U.S., and includes lot numbers and manufacturers (Moderna, Pfizer, and Johnson & Johnson). Healthcare organizations in states without this kind of data sharing don’t have to wait for their state to begin issuing these SMART Health Cards (see below). The design of this open-source standard is decentralized, so that anyone can create the barcodes.
If it’s that easy, what’s the catch?
Right now, the biggest hurdles to overcome are adoption and fragmentation. In answer to the demand for a patient-driven, publicly verifiable, and decentralized proof of vaccination, the Vaccination Credential Initiative defined the open-source SMART Health Card Framework. All the major EHR Vendors, including Epic, Cerner, and Meditech, have adopted the VCI’s design, but businesses still face a patchwork of availability and a diverse public audience.
UCSD was the first U.S. health system to offer patients direct access to secure, verifiable vaccination documentation. The technology is ready, but it’s up to each organization to turn these features on.
How do I know these are real?
The obvious question, then, is how to discern an authentic proof of vaccination. There is more good news: There are apps for that! The Commons Project maintains a list of trusted issuers (such as governments and healthcare organizations) whose public keys can be used to instantly verify the cryptographic signature on the encoded data. Name, date of birth, and immunization details can be displayed and validated without ever collecting or transmitting the individual’s health data.
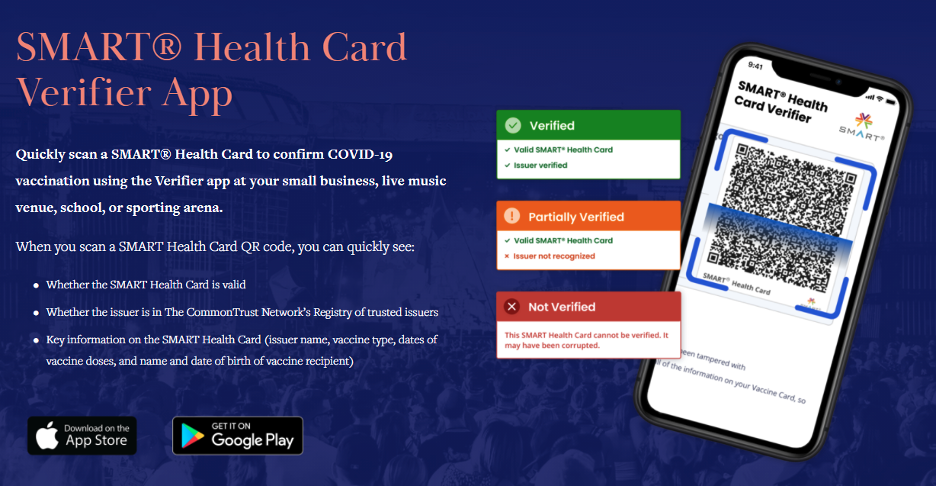
The SMART Health Card standard is extensible and may also be used to provide proof of a recent negative COVID test result. Whether it’s for someone undergoing a high-risk medical procedure or attempting to travel internationally, the same underlying technology can be used to securely authenticate critical health information.
Why do we need a technology solution?
It’s difficult to track how much has changed in the sixteen months since the World Health Organization declared the novel coronavirus (COVID-19) outbreak a global pandemic. For months, testing capacity would lag far behind demand, leaving gaping holes in our first line of defense, especially in underserved and rural communities. Our second line of defense, contact tracing programs, struggled with adoption of tools such as Bluetooth Exposure Notification Systems (ENS). This left many states relying on understaffed county health offices and paper phone books, and by August local governments were overwhelmed.
Technology had been key to the success of public health strategies in places like South Korea, where per-capita case rates and casualties remain near 1/50th what we’ve suffered in the United States. Without these tools, we’re left with imprecise and burdensome interventions that have much larger impacts to the economy and far worse outcomes. If we can make it as easy as possible for health systems to share data with patients, they can focus their efforts on the important stuff: delivering care.